Abstract
Case Report
Individual Treatment Trial of PIGV-Associated Mabry Syndrome with D-Mannose in a Young Child
Marta Agnes Somorai*, Annabelle Arlt, Peter Krawitz, Jochen Baumkötter and Volker Mall
Published: 27 December, 2023 | Volume 6 - Issue 1 | Pages: 001-004
We describe the first individual treatment trial with D-mannose in a young girl with PIGV-CDG. PIGV-CDG belongs to the GPI anchor deficiencies leading to intellectual disability, dysmorphic features, epilepsy, and, less frequently, organ malformations. A hallmark of the GPI anchor deficiencies is the elevated serum alkaline phosphatase (AP). Our patient carried the germline homozygous PIGV variant c.1022C>A, p. (Ala341Glu), the most commonly reported pathogenic variant leading to PIGV-CDG so far. We aimed to improve the impaired enzymatic function of PIGV through elevated substrate levels by giving D-mannose orally. We monitored the clinical status, developmental progress as well as serum AP levels. Our patient experienced no side effects. Standardized developmental testing showed better developmental progress during the 21-month treatment period with D-mannose than in the 12 months following the discontinuation of treatment. The D-Mannose treatment might have had a positive effect on the development of our patient with PIGV-CDG.
Read Full Article HTML DOI: 10.29328/journal.jgmgt.1001008 Cite this Article Read Full Article PDF
Keywords:
PIGV-CDG; D-mannose; GPI-anchor biosynthesis deficiencies; PIGV; HPMRS1; Mabry syndrome; Treatment
References
- Hutny M, Lipinski P, Jezela-Stanek A. Characteristics of Neuroimaging and Behavioural Phenotype in Polish Patients with PIGV-CDG-An Observational Study and Literature Review. Genes (Basel). 2023 May 31;14(6):1208. doi: 10.3390/genes14061208. PMID: 37372388; PMCID: PMC10298423.
- Kang JY, Hong Y, Ashida H, Shishioh N, Murakami Y, Morita YS, Maeda Y, Kinoshita T. PIG-V involved in transferring the second mannose in glycosylphosphatidylinositol. J Biol Chem. 2005 Mar 11;280(10):9489-97. doi: 10.1074/jbc.M413867200. Epub 2004 Dec 28. PMID: 15623507.
- Horn D, Wieczorek D, Metcalfe K, Barić I, Paležac L, Cuk M, Petković Ramadža D, Krüger U, Demuth S, Heinritz W, Linden T, Koenig J, Robinson PN, Krawitz P. Delineation of PIGV mutation spectrum and associated phenotypes in hyperphosphatasia with mental retardation syndrome. Eur J Hum Genet. 2014 Jun;22(6):762-7. doi: 10.1038/ejhg.2013.241. Epub 2013 Oct 16. PMID: 24129430; PMCID: PMC4023216.
- Rodríguez de Los Santos M, Rivalan M, David FS, Stumpf A, Pitsch J, Tsortouktzidis D, Velasquez LM, Voigt A, Schilling K, Mattei D, Long M, Vogt G, Knaus A, Fischer-Zirnsak B, Wittler L, Timmermann B, Robinson PN, Horn D, Mundlos S, Kornak U, Becker AJ, Schmitz D, Winter Y, Krawitz PM. A CRISPR-Cas9-engineered mouse model for GPI-anchor deficiency mirrors human phenotypes and exhibits hippocampal synaptic dysfunctions. Proc Natl Acad Sci U S A. 2021 Jan 12;118(2):e2014481118. doi: 10.1073/pnas.2014481118. PMID: 33402532; PMCID: PMC7812744.
- Horn D, Krawitz P, Mannhardt A, Korenke GC, Meinecke P. Hyperphosphatasia-mental retardation syndrome due to PIGV mutations: expanded clinical spectrum. Am J Med Genet A. 2011 Aug;155A(8):1917-22. doi: 10.1002/ajmg.a.34102. Epub 2011 Jul 7. PMID: 21739589.
- Krawitz PM, Schweiger MR, Rödelsperger C, Marcelis C, Kölsch U, Meisel C, Stephani F, Kinoshita T, Murakami Y, Bauer S, Isau M, Fischer A, Dahl A, Kerick M, Hecht J, Köhler S, Jäger M, Grünhagen J, de Condor BJ, Doelken S, Brunner HG, Meinecke P, Passarge E, Thompson MD, Cole DE, Horn D, Roscioli T, Mundlos S, Robinson PN. Identity-by-descent filtering of exome sequence data identifies PIGV mutations in hyperphosphatasia mental retardation syndrome. Nat Genet. 2010 Oct;42(10):827-9. doi: 10.1038/ng.653. Epub 2010 Aug 29. PMID: 20802478.
- Čechová A, Altassan R, Borgel D, Bruneel A, Correia J, Girard M, Harroche A, Kiec-Wilk B, Mohnike K, Pascreau T, Pawliński Ł, Radenkovic S, Vuillaumier-Barrot S, Aldamiz-Echevarria L, Couce ML, Martins EG, Quelhas D, Morava E, de Lonlay P, Witters P, Honzík T. Consensus guideline for the diagnosis and management of mannose phosphate isomerase-congenital disorder of glycosylation. J Inherit Metab Dis. 2020 Jul;43(4):671-693. doi: 10.1002/jimd.12241. Epub 2020 Apr 21. PMID: 32266963; PMCID: PMC7574589.
- Niehues R, Hasilik M, Alton G, Körner C, Schiebe-Sukumar M, Koch HG, Zimmer KP, Wu R, Harms E, Reiter K, von Figura K, Freeze HH, Harms HK, Marquardt T. Carbohydrate-deficient glycoprotein syndrome type Ib. Phosphomannose isomerase deficiency and mannose therapy. J Clin Invest. 1998 Apr 1;101(7):1414-20. doi: 10.1172/JCI2350. PMID: 9525984; PMCID: PMC508719.
- Taday R, Grüneberg M, DuChesne I, Reunert J, Marquardt T. Dietary mannose supplementation in phosphomannomutase 2 deficiency (PMM2-CDG). Orphanet J Rare Dis. 2020 Sep 22;15(1):258. doi: 10.1186/s13023-020-01528-z. PMID: 32962735; PMCID: PMC7510076.
- Kinoshita T, Fujita M, Maeda Y. Biosynthesis, remodelling and functions of mammalian GPI-anchored proteins: recent progress. J Biochem. 2008 Sep;144(3):287-94. doi: 10.1093/jb/mvn090. Epub 2008 Jul 17. PMID: 18635593.
- Maeda Y, Murakami Y, Kinoshita T. Synthesis, Genetics, and Congenital Diseases of GPI-Anchored Proteins. In: Kanakura Y, Kinoshita T, Nishimura J-I, editors. Paroxysmal Nocturnal Hemoglobinuria: From Bench to Bedside. Tokyo: Springer Japan; 2017;. 11–54.
- Schroeder AS, Kappler M, Bonfert M, Borggraefe I, Schoen C, Reiter K. Seizures and stupor during intravenous mannose therapy in a patient with CDG syndrome type 1b (MPI-CDG). J Inherit Metab Dis. 2010 Dec;33 Suppl 3:S497-502. doi: 10.1007/s10545-010-9252-x. Epub 2011 Jan 16. PMID: 21240668.
- Rani S, Sahai I, Misra M. A Case of Congenital Disorder of Glycosylation Type 1b Presenting as Hyperinsulinemic Hypoglycemia and Failure to Thrive. JCEM Case Rep. 2023 Sep 21;1(5):luad109. doi: 10.1210/jcemcr/luad109. PMID: 37908211; PMCID: PMC10580457.
- De Graef D, Mousa J, Waberski MB, Morava E. Mannose treatment improves immune deficiency in mannose phosphate isomerase-congenital disorder of glycosylation: case report and review of literature. Ther Adv Rare Dis. 2022 Apr 17;3:26330040221091283. doi: 10.1177/26330040221091283. PMID: 37180423; PMCID: PMC10032425.
- Girard M, Douillard C, Debray D, Lacaille F, Schiff M, Vuillaumier-Barrot S, Dupré T, Fabre M, Damaj L, Kuster A, Torre S, Mention K, McLin V, Dobbelaere D, Borgel D, Bauchard E, Seta N, Bruneel A, De Lonlay P. Long term outcome of MPI-CDG patients on D-mannose therapy. J Inherit Metab Dis. 2020 Nov;43(6):1360-1369. doi: 10.1002/jimd.12289. Epub 2020 Aug 9. PMID: 33098580.
Figures:

Figure 1
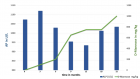
Figure 2
Similar Articles
-
The advances and challenges of Gene Therapy for Duchenne Muscular DystrophyJacques P Tremblay*,Jean-Paul Iyombe-Engembe. The advances and challenges of Gene Therapy for Duchenne Muscular Dystrophy. . 2017 doi: 10.29328/journal.jgmgt.1001003; 1: 019-036
-
Individual Treatment Trial of PIGV-Associated Mabry Syndrome with D-Mannose in a Young ChildMarta Agnes Somorai*, Annabelle Arlt, Peter Krawitz, Jochen Baumkötter, Volker Mall. Individual Treatment Trial of PIGV-Associated Mabry Syndrome with D-Mannose in a Young Child. . 2023 doi: 10.29328/journal.jgmgt.1001008; 6: 001-004
-
Management and Therapeutic Strategies for Spinal Muscular AtrophySheena P Kochumon, Cherupally Krishnan Krishnan Nair*. Management and Therapeutic Strategies for Spinal Muscular Atrophy. . 2024 doi: 10.29328/journal.jgmgt.1001009; 7: 001-007
Recently Viewed
-
Cancer Cell Resistance: The Emergent Intelligence of Adaptation and the Need for Biophysical IntegrationMohamed H Doweidar*. Cancer Cell Resistance: The Emergent Intelligence of Adaptation and the Need for Biophysical Integration. Int J Clin Microbiol Biochem Technol. 2025: doi: 10.29328/journal.ijcmbt.1001031; 8: 007-008
-
Physical Performance in the Overweight/Obesity Children Evaluation and RehabilitationCristina Popescu, Mircea-Sebastian Șerbănescu, Gigi Calin*, Magdalena Rodica Trăistaru. Physical Performance in the Overweight/Obesity Children Evaluation and Rehabilitation. Ann Clin Endocrinol Metabol. 2024: doi: 10.29328/journal.acem.1001030; 8: 004-012
-
Hypercalcaemic Crisis Associated with Hyperthyroidism: A Rare and Challenging PresentationKarthik Baburaj*, Priya Thottiyil Nair, Abeed Hussain, Vimal MV. Hypercalcaemic Crisis Associated with Hyperthyroidism: A Rare and Challenging Presentation. Ann Clin Endocrinol Metabol. 2024: doi: 10.29328/journal.acem.1001029; 8: 001-003
-
Overlap of two unusual condition in childhood: hibernoma and central diabetes insipidusKübra ARSLAN*,Ayça TÖREL ERGÜR,Mehmet Ali YİNANÇ. Overlap of two unusual condition in childhood: hibernoma and central diabetes insipidus. Ann Clin Endocrinol Metabol. 2022: doi: 10.29328/journal.acem.1001023; 6: 001-003
-
Effective treatment of diabetes mellitus and autoimmune diseases by resonance medicinePraznikov Victor*. Effective treatment of diabetes mellitus and autoimmune diseases by resonance medicine. Ann Clin Endocrinol Metabol. 2022: doi: 10.29328/journal.acem.1001024; 6: 004-009
Most Viewed
-
Impact of Latex Sensitization on Asthma and Rhinitis Progression: A Study at Abidjan-Cocody University Hospital - Côte d’Ivoire (Progression of Asthma and Rhinitis related to Latex Sensitization)Dasse Sery Romuald*, KL Siransy, N Koffi, RO Yeboah, EK Nguessan, HA Adou, VP Goran-Kouacou, AU Assi, JY Seri, S Moussa, D Oura, CL Memel, H Koya, E Atoukoula. Impact of Latex Sensitization on Asthma and Rhinitis Progression: A Study at Abidjan-Cocody University Hospital - Côte d’Ivoire (Progression of Asthma and Rhinitis related to Latex Sensitization). Arch Asthma Allergy Immunol. 2024 doi: 10.29328/journal.aaai.1001035; 8: 007-012
-
Causal Link between Human Blood Metabolites and Asthma: An Investigation Using Mendelian RandomizationYong-Qing Zhu, Xiao-Yan Meng, Jing-Hua Yang*. Causal Link between Human Blood Metabolites and Asthma: An Investigation Using Mendelian Randomization. Arch Asthma Allergy Immunol. 2023 doi: 10.29328/journal.aaai.1001032; 7: 012-022
-
An algorithm to safely manage oral food challenge in an office-based setting for children with multiple food allergiesNathalie Cottel,Aïcha Dieme,Véronique Orcel,Yannick Chantran,Mélisande Bourgoin-Heck,Jocelyne Just. An algorithm to safely manage oral food challenge in an office-based setting for children with multiple food allergies. Arch Asthma Allergy Immunol. 2021 doi: 10.29328/journal.aaai.1001027; 5: 030-037
-
Effects of dietary supplementation on progression to type 2 diabetes in subjects with prediabetes: a single center randomized double-blind placebo-controlled trialSathit Niramitmahapanya*,Preeyapat Chattieng,Tiersidh Nasomphan,Korbtham Sathirakul. Effects of dietary supplementation on progression to type 2 diabetes in subjects with prediabetes: a single center randomized double-blind placebo-controlled trial. Ann Clin Endocrinol Metabol. 2023 doi: 10.29328/journal.acem.1001026; 7: 00-007
-
Physical Performance in the Overweight/Obesity Children Evaluation and RehabilitationCristina Popescu, Mircea-Sebastian Șerbănescu, Gigi Calin*, Magdalena Rodica Trăistaru. Physical Performance in the Overweight/Obesity Children Evaluation and Rehabilitation. Ann Clin Endocrinol Metabol. 2024 doi: 10.29328/journal.acem.1001030; 8: 004-012

If you are already a member of our network and need to keep track of any developments regarding a question you have already submitted, click "take me to my Query."