Abstract
Mini Review
Progress in the development of Lipoplex and Polyplex modified with Anionic Polymer for efficient Gene Delivery
Yoshiyuki Hattori*
Published: 24 July, 2017 | Volume 1 - Issue 1 | Pages: 003-018
Nucleic acid-based therapy has become an increasingly important strategy for treating a variety of human diseases. In systemic therapy, a therapeutic gene must be delivered efficiently to its target tissues without side effects. To deliver a therapeutic gene such as plasmid DNA (pDNA) or small interfering RNA (siRNA) to target tissues by systemic administration, cationic carriers such as cationic liposomes and polymers have been commonly used as a non-viral vector. However, the binary complex of therapeutic gene and cationic carrier must be stabilized in the blood circulation by avoiding agglutination with blood components, because electrostatic interactions between positively charged complexes and negatively charged erythrocytes can cause agglutination, and the agglutinates contribute to high entrapment of the therapeutic genes in the highly extended lung capillaries. One promising approach for overcoming this problem is modification of the surface of cationic complexes with anionic biodegradable polymers such as hyaluronic acid, chondroitin sulfate, or polyglutamic acid. As another approach, we recently developed a sequential injection method of anionic polymer and cationic liposome/therapeutic gene complex (cationic lipoplex) for delivery of a therapeutic gene into the liver or liver metastasis. In this review, we describe recent advances in the delivery of therapeutic genes by lipid- and polymer-based carrier systems using anionic polymers.
Read Full Article HTML DOI: 10.29328/journal.jgmgt.1001002 Cite this Article Read Full Article PDF
References
- Collins M, Thrasher AA. Gene therapy: progress and predictions. Proc Biol Sci. 2015; 282. Ref.: https://goo.gl/MpvdAg
- Aagaard L, Rossi JJ. RNAi therapeutics: principles, prospects and challenges. Adv Drug Deliv Rev. 2007; 59: 75-86. Ref.: https://goo.gl/w6Ljep
- He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet. 2004; 5: 522-531. Ref.: https://goo.gl/xtPsZE
- Wang H, Jiang Y, Peng H, Chen Y, Zhu P, et al. Recent progress in microRNA delivery for cancer therapy by non-viral synthetic vectors. Adv Drug Deliv Rev. 2015; 81: 142-160. Ref.: https://goo.gl/pr7ZdH
- Zhang Y, Wang Z, Gemeinhart RA. Progress in microRNA delivery. J Control Release. 2013; 172: 962-974. Ref.: https://goo.gl/ZFRb69
- Roberts TC, Ezzat K, El Andaloussi S, Weinberg MS. Synthetic SiRNA Delivery: Progress and Prospects. Methods Mol Biol. 2016; 1364: 291-310. Ref.: https://goo.gl/WDZAmf
- Young LS, Searle PF, Onion D, Mautner V. Viral gene therapy strategies: from basic science to clinical application, J Pathol. 2006; 208: 299-318. Ref.: https://goo.gl/uvg8Ay
- Kaiser J. Gene therapy. Seeking the cause of induced leukemias in X-SCID trial. Science. 2003; 299: 495. Ref.: https://goo.gl/nsHKvE
- Yin H, Kanasty RL, Eltoukhy AA, Vegas AJ, Dorkin JR, et al. Non-viral vectors for gene-based therapy. Nat Rev Genet. 2014; 15: 541-555. Ref.: https://goo.gl/zRNE5K
- Glover DJ, Lipps HJ, Jans DA. Towards safe, non-viral therapeutic gene expression in humans. Nat Rev Genet. 2005; 6: 299-310. Ref.: https://goo.gl/Dp8a7i
- Zhou J, Shum KT, Burnett JC, Rossi JJ. Nanoparticle-Based Delivery of RNAi Therapeutics: Progress and Challenges. Pharmaceuticals. 2013; 6: 85-107. Ref.: https://goo.gl/eXwyQM
- Zhang S, Zhi D, Huang L. Lipid-based vectors for siRNA delivery. J Drug Target. 2012; 20: 724-735. Ref.: https://goo.gl/QjH3mf
- Hwang SJ, Davis ME. Cationic polymers for gene delivery: designs for overcoming barriers to systemic administration. Curr Opin Mol Ther. 2001; 3: 183-191. Ref.: https://goo.gl/QXaq9z
- Goula D, Benoist C, Mantero S, Merlo G, Levi G, et al. Polyethylenimine-based intravenous delivery of transgenes to mouse lung. Gene Ther. 1998; 5: 1291-1295. Ref.: https://goo.gl/4Jkmpk
- Yeeprae W, Kawakami S, Suzuki S, Yamashita F, Hashida M. Physicochemical and pharmacokinetic characteristics of cationic liposomes. Pharmazie. 2006; 61: 102-105. Ref.: https://goo.gl/bFxs41
- Eliyahu H, Servel N, Domb AJ, Barenholz Y. Lipoplex-induced hemagglutination: potential involvement in intravenous gene delivery. Gene Ther. 2002; 9: 850-858. Ref.: https://goo.gl/npqi3U
- Simberg D, Weisman S, Talmon Y, Faerman A, Shoshani T, et al. The role of organ vascularization and lipoplex-serum initial contact in intravenous murine lipofection. J Biol Chem. 2003; 278: 39858-39865. Ref.: https://goo.gl/W8gqip
- 18 Y. Hattori. Delivery of plasmid DNA into tumors by intravenous injection of PEGylated cationic lipoplexes into tumor-bearing mice. Pharmacology & Pharmacy. 2016; 7.
- Gjetting T, Arildsen NS, Christensen CL, Poulsen TT, Roth JA, et al. In vitro and in vivo effects of polyethylene glycol (PEG)-modified lipid in DOTAP/cholesterol-mediated gene transfection. Int J Nanomedicine. 2010; 5: 371-383. Ref.: https://goo.gl/wLcjFt
- Bourguignon LY. Hyaluronan-mediated CD44 activation of RhoGTPase signaling and cytoskeleton function promotes tumor progression. Semin Cancer Biol. 2008; 18: 251-259. Ref.: https://goo.gl/9xxva6
- Cortes-Dericks L, Schmid RA. CD44 and its ligand hyaluronan as potential biomarkers in malignant pleural mesothelioma: evidence and perspectives. Respir Res. 2017; 18: 58. Ref.: https://goo.gl/6SvFda
- Toole BP. Hyaluronan-CD44 Interactions in Cancer: Paradoxes and Possibilities. Clin Cancer Res. 2009; 15: 7462-7468. Ref.: https://goo.gl/pcCFMU
- Xie F, Zhang L, Peng J, Li C1, Pu J, et al. Hepatic Carcinoma Selective Nucleic Acid Nanovector Assembled by Endogenous Molecules Based on Modular Strategy. Mol Pharm. 2017; 14: 1841-1851. Ref.: https://goo.gl/td4jGT
- Chen M, Zeng Z, Qu X, Tang Y, Long Q, et al. Biocompatible anionic polyelectrolyte for improved liposome based gene transfection. Int J Pharm. 2015; 490: 173-179. Ref.: https://goo.gl/bHhj68
- Lu HD, Zhao HQ, Wang K, Lv LL. Novel hyaluronic acid-chitosan nanoparticles as non-viral gene delivery vectors targeting osteoarthritis. Int J Pharm. 2011; 420: 358-365. Ref.: https://goo.gl/ynnqri
- Lu H, Lv L, Dai Y, Wu G, Zhao H, et al. Porous chitosan scaffolds with embedded hyaluronic acid/chitosan/plasmid-DNA nanoparticles encoding TGF-β1 induce DNA controlled release, transfected chondrocytes, and promoted cell proliferation. PloS one. 2013; 8. Ref.: https://goo.gl/rpgcwQ
- Raviña M, Cubillo E, Olmeda D, Novoa-Carballal R, Fernandez-Megia E, et al. Hyaluronic acid/chitosan-g-poly(ethylene glycol) nanoparticles for gene therapy: an application for pDNA and siRNA delivery. Pharm Res. 2010; 27: 2544-2555. Ref.: https://goo.gl/JMcnXV
- Ito T, Koyama Y, Otsuka M, et al. Analysis of the surface structure of DNA/polycation/hyaluronic acid ternary complex by Raman microscopy. J Pharm Biomed Anal. 2010; 51: 268-272. Ref.: https://goo.gl/8TTMxc
- Ito T, Koyama Y, Otsuka M. Preparation of calcium phosphate nanocapsule including deoxyribonucleic acid-polyethyleneimine-hyaluronic acid ternary complex for durable gene delivery. J Pharm Sci. 2014; 103: 179-184. Ref.: https://goo.gl/tevmrh
- Koyama Y, Sugiura K, Yoshihara C, Inaba T, Ito T. Highly Effective Non-Viral Antitumor Gene Therapy System Comprised of Biocompatible Small Plasmid Complex Particles Consisting of pDNA, Anionic Polysaccharide, and Fully Deprotected Linear Polyethylenimine. Pharmaceutics. 2015; 7: 152-164. Ref.: https://goo.gl/J2CNsp
- Li Y, Zhang J, Wang B, Shen Y, Ouahab A. Co-delivery of siRNA and hypericin into cancer cells by hyaluronic acid modified PLGA-PEI nanoparticles. Drug Dev Ind Pharm. 2016; 42: 737-746. Ref.: https://goo.gl/T1JGhu
- Zhao MD, Cheng JL, Yan JJ, Chen FY, Sheng JZ, et al. Hyaluronic acid reagent functional chitosan-PEI conjugate with AQP2-siRNA suppressed endometriotic lesion formation. Int J Nanomedicine. 2016; 11: 1323-1336. Ref.: https://goo.gl/5LQk7N
- Chen CJ, Zhao ZX, Wang JC, Zhao EY, Gao LY, et al. A comparative study of three ternary complexes prepared in different mixing orders of siRNA/redox-responsive hyperbranched poly (amido amine)/hyaluronic acid. Int J Nanomedicine. 2012; 7: 3837-3849. Ref.: https://goo.gl/FEtQ2n
- Gu J, Chen X, Ren X, Zhang X, Fang X, et al. CD44-Targeted Hyaluronic Acid-Coated Redox-Responsive Hyperbranched Poly(amido amine)/Plasmid DNA Ternary Nanoassemblies for Efficient Gene Delivery. Bioconjug Chem. 2016; 27: 1723-1736. Ref.: https://goo.gl/WA48jv
- Kim EJ, Shim G, Kim K, Kwon IC, Oh YK, et al. Hyaluronic acid complexed to biodegradable poly L-arginine for targeted delivery of siRNAs. The J Gene Med. 2009; 11: 791-803. Ref.: https://goo.gl/2cmfJb
- Surace C, Arpicco S, Dufaÿ-Wojcicki A, Marsaud V, Bouclier C, et al. Lipoplexes targeting the CD44 hyaluronic acid receptor for efficient transfection of breast cancer cells. Mol Pharm. 2009; 6: 1062-1073. Ref.: https://goo.gl/rTUoFA
- Dufaÿ Wojcicki A, Hillaireau H, Nascimento TL, Arpicco S, Taverna M, et al. Hyaluronic acid-bearing lipoplexes: physico-chemical characterization and in vitro targeting of the CD44 receptor. J Control Release. 2012; 162: 545-552. Ref.: https://goo.gl/DEP7mL
- Leite Nascimento T, Hillaireau H, Vergnaud J, Rivano M, Deloménie C, et al. Hyaluronic acid-conjugated lipoplexes for targeted delivery of siRNA in a murine metastatic lung cancer model. Int J Pharm. 2016; 514: 103-111. Ref.: https://goo.gl/Ztr3q5
- Nascimento TL, Hillaireau H, Noiray M, Bourgaux C, Arpicco S, et al. Supramolecular Organization and siRNA Binding of Hyaluronic Acid-Coated Lipoplexes for Targeted Delivery to the CD44 Receptor. Langmuir. 2015; 31: 11186-11194. Ref.: https://goo.gl/QwyYoY
- Taetz S, Bochot A, Surace C, Arpicco S, Renoir JM, et al. Hyaluronic acid-modified DOTAP/DOPE liposomes for the targeted delivery of anti-telomerase siRNA to CD44-expressing lung cancer cells. Oligonucleotides. 2009; 19: 103-116. Ref.: https://goo.gl/4XksiJ
- Yang X, Iyer AK, Singh A, Milane L, Choy E, et al. Cluster of Differentiation 44 Targeted Hyaluronic Acid Based Nanoparticles for MDR1 siRNA Delivery to Overcome Drug Resistance in Ovarian Cancer. Pharm Res. 2015; 32: 2097-2109. Ref.: https://goo.gl/Vamt93
- Jiang G, Park K, Kim J, Kim KS, Oh EJ, et al. Hyaluronic acid-polyethyleneimine conjugate for target specific intracellular delivery of siRNA. Biopolymers. 2008; 89: 635-642. Ref.: https://goo.gl/D7PuEF
- Park K, Lee MY, Kim KS, Hahn SK. Target specific tumor treatment by VEGF siRNA complexed with reducible polyethyleneimine-hyaluronic acid conjugate. Biomaterials. 2010; 31: 5258-5265. Ref.: https://goo.gl/GwUTLX
- Ganesh S, Iyer AK, Morrissey DV, Amiji MM. Hyaluronic acid based self-assembling nanosystems for CD44 target mediated siRNA delivery to solid tumors. Biomaterials. 2013; 34: 3489-3502. Ref.: https://goo.gl/5NYVTw
- Han SE, Kang H, Shim GY, Kim SJ, Choi HG, et al. Cationic derivatives of biocompatible hyaluronic acids for delivery of siRNA and antisense oligonucleotides. J Drug Target. 2009; 17: 123-132. Ref.: https://goo.gl/FysFvE
- Tran TH, Rastogi R, Shelke J, Amiji MM. Modulation of Macrophage Functional Polarity towards Anti-Inflammatory Phenotype with Plasmid DNA Delivery in CD44 Targeting Hyaluronic Acid Nanoparticles. Sci Rep. 2015; 5: 16632. Ref.: https://goo.gl/DXrNH8
- Tran TH, Krishnan S, Amiji MM. MicroRNA-223 Induced Repolarization of Peritoneal Macrophages Using CD44 Targeting Hyaluronic Acid Nanoparticles for Anti-Inflammatory Effects. PloS one. 2016; 11. Ref.: https://goo.gl/yujGhm
- Lee MS, Lee JE, Byun E, Kim NW, Lee K, et al. Target-specific delivery of siRNA by stabilized calcium phosphate nanoparticles using dopa-hyaluronic acid conjugate. J Control Release. 2014; 192: 122-130. Ref.: https://goo.gl/9BdWDp
- Jang YL, Ku SH, Jin S, Park JH, Kim WJ, et al. Hyaluronic acid-siRNA conjugate/reducible polyethylenimine complexes for targeted siRNA delivery. J Nanosci Nanotechnol. 2014; 14: 7388-7394. Ref.: https://goo.gl/yQ7KsP
- Mok H, Park JW, Park TG. Antisense oligodeoxynucleotide-conjugated hyaluronic acid/protamine nanocomplexes for intracellular gene inhibition. Bioconjug Chem. 2007; 18: 1483-1489. Ref.: https://goo.gl/AqgFzn
- Mizumoto S, Sugahara K. Glycosaminoglycans are functional ligands for receptor for advanced glycation end-products in tumors. FEBS J. 2013; 280: 2462-2470. Ref.: https://goo.gl/6WXTEh
- Henrotin Y, Mathy M, Sanchez C, Lambert C. Chondroitin sulfate in the treatment of osteoarthritis: from in vitro studies to clinical recommendations. Ther Adv Musculoskelet Dis. 2010; 2: 335-348. Ref.: https://goo.gl/bN4A1J
- Uchida S, Itaka K, Chen Q, Osada K, Miyata K, et al. T. Combination of chondroitin sulfate and polyplex micelles from Poly(ethylene glycol)-poly{N'-[N-(2-aminoethyl)-2-aminoethyl]aspartamide} block copolymer for prolonged in vivo gene transfection with reduced toxicity. J Control Release. 2011; 155: 296-302.
- Hattori Y, Yamasaku H, Maitani Y. Anionic polymer-coated lipoplex for safe gene delivery into tumor by systemic injection. J Drug Target. 2013; 21: 639-647. Ref.: https://goo.gl/YnRp23
- Hattori Y, Arai S, Kikuchi T, Ozaki KI, Kawano K, et al. Therapeutic effect for liver-metastasized tumor by sequential intravenous injection of anionic polymer and cationic lipoplex of siRNA. J Drug Target. 2016; 24: 309-317. Ref.: https://goo.gl/HaoJZ1
- Lo YL, Sung KH, Chiu CC, Wang LF. Chemically conjugating polyethylenimine with chondroitin sulfate to promote CD44-mediated endocytosis for gene delivery. Mol Pharm. 2013; 10: 664-676. Ref.: https://goo.gl/hXHZmc
- Liang S, Duan Y, Xing Z, Han H, Zhang A, et al. Inhibition of cell proliferation and migration by chondroitin sulfate-g-polyethylenimine-mediated miR-34a delivery. Colloids Surf B Biointerfaces. 2015; 136: 577-584. Ref.: https://goo.gl/VcoikX
- Chen W, Liu Y, Liang X, Huang Y, Li Q. Chondroitin sulfate-functionalized polyamidoamine as a tumor-targeted carrier for miR-34a delivery. Acta biomater. 2017; 57: 238-250. Ref.: https://goo.gl/2VpD2E
- Kurosaki T, Kitahara T, Fumoto S, Nishida K, Yamamoto K, et al. Chondroitin sulfate capsule system for efficient and secure gene delivery. J Pharm Pharm Sci. 2010; 13: 351-361. Ref.: https://goo.gl/36Rj1T
- Hagiwara K, Nakata M, Koyama Y, Sato T. The effects of coating pDNA/chitosan complexes with chondroitin sulfate on physicochemical characteristics and cell transfection. Biomaterials. 2012; 33: 7251-7260. Ref.: https://goo.gl/PTwfKF
- Hagiwara K, Kishimoto S, Ishihara M, Koyama Y, Mazda O, et al. In vivo gene transfer using pDNA/chitosan/chondroitin sulfate ternary complexes: influence of chondroitin sulfate on the stability of freeze-dried complexes and transgene expression in vivo. J Gene Med. 2013; 15: 83-92. Ref.: https://goo.gl/Vbktx4
- Hamada K, Yoshihara C, Ito T, Tani K, Tagawa M, et al. Antitumor effect of chondroitin sulfate-coated ternary granulocyte macrophage-colony-stimulating factor plasmid complex for ovarian cancer. J Gene Med. 2012; 14: 120-127. Ref.: https://goo.gl/sc2j3a
- Iwanaga M, Kodama Y, Muro T, Nakagawa H, Kurosaki T, et al. Biocompatible complex coated with glycosaminoglycan for gene delivery. J Drug Target. 2017; 25: 370-378. Ref.: https://goo.gl/23rjZJ
- Imamura M, Kodama Y, Higuchi N, Kanda K, Nakagawa H, et al. Ternary complex of plasmid DNA electrostatically assembled with polyamidoamine dendrimer and chondroitin sulfate for effective and secure gene delivery. Biol Pharm Bull. 2014; 37: 552-559. Ref.: https://goo.gl/mrgDuM
- Kurosaki T, Uematsu M, Shimoda K, Suzuma K, Nakai M, et al. Ocular gene delivery systems using ternary complexes of plasmid DNA, polyethylenimine, and anionic polymers. Biol Pharm Bull. 2013; 36: 96-101. Ref.: https://goo.gl/asDxcu
- Liao ZX, Peng SF, Ho YC, Mi FL, Maiti B, et al. Mechanistic study of transfection of chitosan/DNA complexes coated by anionic poly(gamma-glutamic acid). Biomaterials. 2012; 33: 3306-3315. Ref.: https://goo.gl/4zW749
- Peng SF, Tseng MT, Ho YC, Wei MC, Liao ZX, et al. Mechanisms of cellular uptake and intracellular trafficking with chitosan/DNA/poly(gamma-glutamic acid) complexes as a gene delivery vector. Biomaterials. 2011; 32: 239-248. Ref.: https://goo.gl/amcfS3
- Hanigan MH, Ricketts WA. Extracellular glutathione is a source of cysteine for cells that express gamma-glutamyl transpeptidase. Biochemistry. 1993; 32: 6302-6306. Ref.: https://goo.gl/cvbf71
- Pompella A, De Tata V, Paolicchi A, Zunino F. Expression of gamma-glutamyltransferase in cancer cells and its significance in drug resistance. Biochem Pharmacol. 2006; 71: 231-238. Ref.: https://goo.gl/PXag69
- Schafer C, Fels C, Brucke M, Holzhausen HJ, Bahn H, et al. Gamma-glutamyl transferase expression in higher-grade astrocytic glioma. Acta Oncol. 2001; 40: 529-535. Ref.: https://goo.gl/YneGs5
- Yao D, Jiang D, Huang Z, Lu J, Tao Q, et al. Abnormal expression of hepatoma specific gamma-glutamyl transferase and alteration of gamma-glutamyl transferase gene methylation status in patients with hepatocellular carcinoma. Cancer. 2000; 88: 761-769. Ref.: https://goo.gl/9AhhC2
- Hanigan MH, Frierson HF Jr, Brown JE, Lovell MA, Taylor PT. Human ovarian tumors express gamma-glutamyl transpeptidase. Cancer Res. 1994; 54: 286-290. Ref.: https://goo.gl/PWbnX5
- Liao ZX, Ho YC, Chen HL, Peng SF, Hsiao CW, et al. Enhancement of efficiencies of the cellular uptake and gene silencing of chitosan/siRNA complexes via the inclusion of a negatively charged poly(gamma-glutamic acid). Biomaterials. 2010; 31: 8780-8788. Ref.: https://goo.gl/K4fK1H
- Kodama Y, Kuramoto H, Mieda Y, Muro T, Nakagawa H, et al. Application of biodegradable dendrigraft poly-l-lysine to a small interfering RNA delivery system. J Drug Target. 2017; 25: 49-57. Ref.: https://goo.gl/M7pyVt
- Kanda K, Kodama Y, Kurosaki T, Imamura M, Nakagawa H, et al. Ternary complex of plasmid DNA with protamine and gamma-polyglutamic acid for biocompatible gene delivery system. Biol Pharm Bull. 2013; 36: 1794-1799. Ref.: https://goo.gl/bBRTNr
- Kodama Y, Shiokawa Y, Nakamura T, Kurosaki T, Aki K, et al. Novel siRNA delivery system using a ternary polymer complex with strong silencing effect and no cytotoxicity. Biol Pharm Bull. 2014; 37: 1274-1281. Ref.: https://goo.gl/L61STv
- Hattori Y, Nakamura A, Arai S, Nishigaki M, Ohkura H, et al. In vivo siRNA delivery system for targeting to the liver by poly-l-glutamic acid-coated lipoplex, Results Pharma Sci. 2014; 4: 1-7. Ref.: https://goo.gl/96eSUj
- Schlegel A, Largeau C, Bigey P, Bessodes M, Lebozec K, et al. Anionic polymers for decreased toxicity and enhanced in vivo delivery of siRNA complexed with cationic liposomes. J Control Release. 2011; 152: 393-401. Ref.: https://goo.gl/uQDVxW
- Schlegel A, Bigey P, Dhotel H, Scherman D, Escriou V. Reduced in vitro and in vivo toxicity of siRNA-lipoplexes with addition of polyglutamate. J Control Release. 2013; 165: 1-8. Ref.: https://goo.gl/tZHkSo
- Kodama Y, Nakamura T, Kurosaki T, Egashira K, Mine T, et al. Biodegradable nanoparticles composed of dendrigraft poly-L-lysine for gene delivery. Eur J Pharm Biopharm. 2014; 87: 472-479. Ref.: https://goo.gl/7ghqB4
- Kurosaki T, Kitahara T, Fumoto S, Nishida K, Nakamura J, et al. Ternary complexes of pDNA, polyethylenimine, and gamma-polyglutamic acid for gene delivery systems. Biomaterials. 2009; 30: 2846-2853. Ref.: https://goo.gl/CEjceW
- Kurosaki T, Kodama Y, Muro T, Higuchi N, Nakamura T, et al. Secure splenic delivery of plasmid DNA and its application to DNA vaccine. Biol Pharm Bull. 2013; 36: 1800-1806. Ref.: https://goo.gl/sPQjwY
- Tripathi SK, Goyal R, Ansari KM, Ravi Ram K, Shukla Y, et al. Polyglutamic acid-based nanocomposites as efficient non-viral gene carriers in vitro and in vivo. Eur J Pharm Biopharm. 2011; 79: 473-484. Ref.: https://goo.gl/9m2ySs
- Boyle WS, Senger K, Tolar J, Reineke TM. Heparin Enhances Transfection in Concert with a Trehalose-Based Polycation with Challenging Cell Types. Biomacromolecules. 2017; 18: 56-67. Ref.: https://goo.gl/6PyzkG
- Zhou X, Li X, Gou M, Qiu J, Li J,et al. Antitumoral efficacy by systemic delivery of heparin conjugated polyethylenimine-plasmid interleukin-15 complexes in murine models of lung metastasis. Cancer Sci. 2011; 102: 1403-1409. Ref.: https://goo.gl/hLQiCc
- Hattori Y, Arai S, Okamoto R, Hamada M, Kawano K, et al. Sequential intravenous injection of anionic polymer and cationic lipoplex of siRNA could effectively deliver siRNA to the liver. Int J Pharm. 2014; 476: 289-298. Ref.: https://goo.gl/ojVgE1
- Hattori Y, Arai S, Kikuchi T, Hamada M, Okamoto R, et al. Optimization of siRNA delivery method into the liver by sequential injection of polyglutamic acid and cationic lipoplex. Pharmacol & Pharma. 2015; 6: 302-310. Ref.: https://goo.gl/K6JkPs
- Hattori Y, Yoshiike Y, Kikuchi T, Yamamoto N, Ozaki K, et al. Evaluation of injection route of anionic polymer for siRNA delivery into the liver by sequential injection of anionic polymer and cationic lipoplex of siRNA. J. Drug Deliv. Sci. Tec. 2016; 35: 40-49. Ref.: https://goo.gl/VmbwSG
- Tan GK, Tabata Y. Chondroitin-6-sulfate attenuates inflammatory responses in murine macrophages via suppression of NF-kappaB nuclear translocation. Acta Biomater. 2014; 10: 2684-2692. Ref.: https://goo.gl/uj47Ym
- Stabler TV, Huang Z, Montell E, Vergés J, Kraus VB. Chondroitin sulphate inhibits NF-κB activity induced by interaction of pathogenic and damage associated molecules. Osteoarthritis Cartilage. 2017; 25: 166-174. Ref.: https://goo.gl/3GAnRV
- Hattori Y, Kikuchi T, Nakamura M, Ozaki K, Onishi H. Therapeutic effects of protein kinase N3 small interfering RNA and doxorubicin combination therapy on liver and lung metastases. Oncol Lett, in press.
Figures:

Figure 1
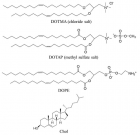
Figure 2
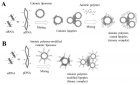
Figure 3
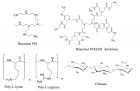
Figure 4

Figure 5
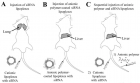
Figure 6
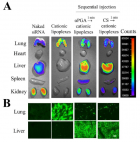
Figure 7
Similar Articles
-
CRISPR genome editing: A general viewRam Mohan Ram Kumar*. CRISPR genome editing: A general view. . 2017 doi: 10.29328/journal.jgmgt.1001001; 1: 001-002
-
Progress in the development of Lipoplex and Polyplex modified with Anionic Polymer for efficient Gene DeliveryYoshiyuki Hattori*. Progress in the development of Lipoplex and Polyplex modified with Anionic Polymer for efficient Gene Delivery. . 2017 doi: 10.29328/journal.jgmgt.1001002; 1: 003-018
-
The advances and challenges of Gene Therapy for Duchenne Muscular DystrophyJacques P Tremblay*,Jean-Paul Iyombe-Engembe. The advances and challenges of Gene Therapy for Duchenne Muscular Dystrophy. . 2017 doi: 10.29328/journal.jgmgt.1001003; 1: 019-036
-
The role of genetic mutations in genes LMNA, PPARG, PLIN1, AKT2, CIDEC in Köbberling–Dunnigan SyndromeShahin Asadi*,Mahsa Jamali. The role of genetic mutations in genes LMNA, PPARG, PLIN1, AKT2, CIDEC in Köbberling–Dunnigan Syndrome. . 2019 doi: 10.29328/journal.jgmgt.1001004; 2: 001-006
-
Reasons why new coronavirus, SARS-CoV-2 infections are likely to spreadTakuma Hayashi*,Takashi Ura,Kaoru Abiko,Masaki Mandan,Nobuo Yaegashi,Ikuo Konishi. Reasons why new coronavirus, SARS-CoV-2 infections are likely to spread. . 2020 doi: 10.29328/journal.jgmgt.1001005; 3: 001-003
-
Partial SHOX duplications associated with various cases of congenital uterovaginal aplasia (MRKH syndrome): A tangible evidence but a puzzling mechanismDaniel Guerrier*,Karine Morcel. Partial SHOX duplications associated with various cases of congenital uterovaginal aplasia (MRKH syndrome): A tangible evidence but a puzzling mechanism. . 2021 doi: 10.29328/journal.jgmgt.1001006; 4: 001-008
-
New insights of liquid biopsy in ovarian cancerPanagiotis Antoniadis*,Florentina Alina Gheorghe,Madalina Ana Maria Nitu,Cezara Gabriela Nitu,Diana Roxana Constantinescu,Florentina Duica. New insights of liquid biopsy in ovarian cancer. . 2022 doi: 10.29328/journal.jgmgt.1001007; 5: 001-011
-
Individual Treatment Trial of PIGV-Associated Mabry Syndrome with D-Mannose in a Young ChildMarta Agnes Somorai*, Annabelle Arlt, Peter Krawitz, Jochen Baumkötter, Volker Mall. Individual Treatment Trial of PIGV-Associated Mabry Syndrome with D-Mannose in a Young Child. . 2023 doi: 10.29328/journal.jgmgt.1001008; 6: 001-004
-
Management and Therapeutic Strategies for Spinal Muscular AtrophySheena P Kochumon, Cherupally Krishnan Krishnan Nair*. Management and Therapeutic Strategies for Spinal Muscular Atrophy. . 2024 doi: 10.29328/journal.jgmgt.1001009; 7: 001-007
-
A Critical Review on Some Recent Developments in Comparison of Biological SequencesDK Bhattacharya*. A Critical Review on Some Recent Developments in Comparison of Biological Sequences. . 2024 doi: 10.29328/journal.jgmgt.1001010; 7: 008-014
Recently Viewed
-
Unveiling Disparities in WHO Grade II Glioma Care among Physicians in Middle East and North African (MENA) Countries: A Multidisciplinary SurveyFatimah M Kaabi,Layth Mula-Hussain*,Shakir Al-Shakir,Sultan Alsaiari,Leonidas Chelis,Renda AlHabib,Sara Owaidah,Renad Subaie,Marwah M Abdulkader,Ibrahim Alotain. Unveiling Disparities in WHO Grade II Glioma Care among Physicians in Middle East and North African (MENA) Countries: A Multidisciplinary Survey. Arch Cancer Sci Ther. 2026: doi: 10.29328/journal.acst.1001048; 10: 001-005
-
Knowledge, Attitude, and Practice of Healthcare Workers in Ekiti State, Nigeria on Prevention of Cervical CancerAde-Ojo Idowu Pius*, Okunola Temitope Omoladun, Olaogun Dominic Oluwole. Knowledge, Attitude, and Practice of Healthcare Workers in Ekiti State, Nigeria on Prevention of Cervical Cancer. Arch Cancer Sci Ther. 2024: doi: 10.29328/journal.acst.1001038; 8: 001-006
-
Oral Cancer Management is not just Treatment! But also, how early Pre-cancerous Lesions are Diagnosed & Treated!!Suresh Kishanrao*. Oral Cancer Management is not just Treatment! But also, how early Pre-cancerous Lesions are Diagnosed & Treated!!. Arch Cancer Sci Ther. 2024: doi: 10.29328/journal.acst.1001039; 8: 007-012
-
Lived Experiences of Cervical Cancer Patients Receiving Chemotherapy at Cancer Diseases Hospital in Lusaka, ZambiaElisha Benkeni Kapya*, Marjorie Kabinga Makukula, Mwaba Chileshe Siwale, Victoria Kalusopa Mwiinga, Elijah Mpundu. Lived Experiences of Cervical Cancer Patients Receiving Chemotherapy at Cancer Diseases Hospital in Lusaka, Zambia. Arch Cancer Sci Ther. 2024: doi: 10.29328/journal.acst.1001041; 8: 019-036
-
Breast Cancer in FemaleLorena Menditto*. Breast Cancer in Female. Arch Cancer Sci Ther. 2024: doi: 10.29328/journal.acst.1001040; 8: 013-018
Most Viewed
-
Effects of dietary supplementation on progression to type 2 diabetes in subjects with prediabetes: a single center randomized double-blind placebo-controlled trialSathit Niramitmahapanya*,Preeyapat Chattieng,Tiersidh Nasomphan,Korbtham Sathirakul. Effects of dietary supplementation on progression to type 2 diabetes in subjects with prediabetes: a single center randomized double-blind placebo-controlled trial. Ann Clin Endocrinol Metabol. 2023 doi: 10.29328/journal.acem.1001026; 7: 00-007
-
Physical Performance in the Overweight/Obesity Children Evaluation and RehabilitationCristina Popescu, Mircea-Sebastian Șerbănescu, Gigi Calin*, Magdalena Rodica Trăistaru. Physical Performance in the Overweight/Obesity Children Evaluation and Rehabilitation. Ann Clin Endocrinol Metabol. 2024 doi: 10.29328/journal.acem.1001030; 8: 004-012
-
Hypercalcaemic Crisis Associated with Hyperthyroidism: A Rare and Challenging PresentationKarthik Baburaj*, Priya Thottiyil Nair, Abeed Hussain, Vimal MV. Hypercalcaemic Crisis Associated with Hyperthyroidism: A Rare and Challenging Presentation. Ann Clin Endocrinol Metabol. 2024 doi: 10.29328/journal.acem.1001029; 8: 001-003
-
Exceptional cancer responders: A zone-to-goDaniel Gandia,Cecilia Suárez*. Exceptional cancer responders: A zone-to-go. Arch Cancer Sci Ther. 2023 doi: 10.29328/journal.acst.1001033; 7: 001-002
-
The benefits of biochemical bone markersSek Aksaranugraha*. The benefits of biochemical bone markers. Int J Bone Marrow Res. 2020 doi: 10.29328/journal.ijbmr.1001013; 3: 027-031

If you are already a member of our network and need to keep track of any developments regarding a question you have already submitted, click "take me to my Query."